- Waskyra™ is developed by Fondazione Telethon for Wiskott-Aldrich syndrome.
- Wiskott-Aldrich syndrome affects 1 in 250,000 live male births.
- AGC Biologics provided support for lentiviral vector production and regulatory compliance.
Approval of Waskyra™
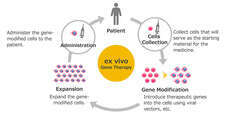
AGC Biologics announced that Waskyra™, an ex vivo gene therapy for Wiskott-Aldrich syndrome, has received marketing authorization from the U.S. FDA and the European Commission. Developed by Fondazione Telethon, Waskyra™ offers a new treatment option for this rare immune disorder.
Wiskott-Aldrich Syndrome
Wiskott-Aldrich syndrome is a rare immune disorder affecting approximately one in 250,000 live male births. Traditional treatments have included symptomatic therapies and hematopoietic stem cell transplants from family donors.
Innovative Treatment Approach
Waskyra™ uses the patient's own cells to correct the genetic defect, reducing donor dependency and rejection risk, while aiming for a curative outcome with minimal burden.
AGC Biologics' Role
AGC Biologics leveraged its expertise in process development and contract manufacturing of gene and cell therapies. The company provided comprehensive support for lentiviral vector production, patient-specific genetically modified cell manufacturing, and regulatory compliance, assisting with regulatory filings at every step.

